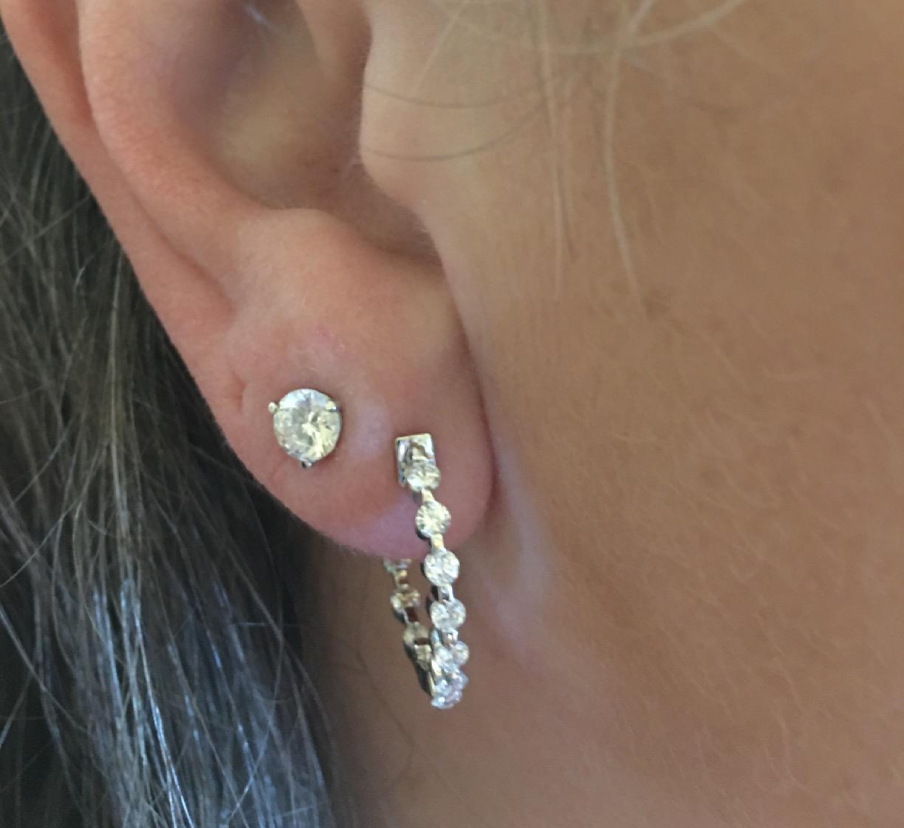
lab-diamond-martini-studs
Translation missing: en.gempages.Product.product_not_found
Translation missing: en.gempages.Product.product_not_found

Welcome to Shilat Jewelers, where luxury meets sustainability. Explore our exquisite collection of lab-grown diamond stud earrings, meticulously crafted to captivate and inspire.
At Shilat Jewelers, we believe in the power of elegance and responsibility. That's why our lab-grown diamond stud earrings are not only stunning but also environmentally friendly and ethically sourced.
Translation missing: en.gempages.Product.product_not_found

Why choose lab-grown diamond stud earrings ?

Our Commitment to Quality and Sustainability

Customer Satisfaction Guaranteed

Shop with Confidence

Sustainable Luxury

Craftsmanship and Quality
9,942+
99.6%
20+Years
77+






Who are we